Light gasses
Hydrogen Storage in Carbon Based Materials
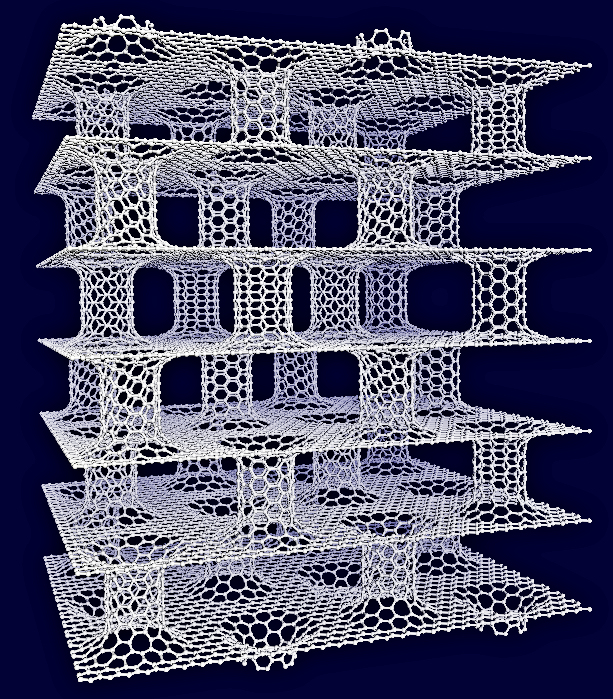
Carbon Based Materials are considered ideal candidates for hydrogen storage due to their light weight and structural stability. However, existing carbon materials, like Carbon Nanotubes (CNTs), do not satisfy H2 storage capacity targets.
Existing H2 storage technologies (metal hydrides, cryogenic conditions and high pressure tanks) are inefficient for automobile applications, for a variety of reasons. A radical solution to the hydrogen storage problem would be the design of new, tailor–made, compact, light nanomaterials that can sorb H2 at ambient conditions.
Having in mind carbon’s superior structural stability, low–weight and ease of processing, we aimed to design a carbon–based material with tunable pores and high gravimetric and volumetric H2 storage capacity. The objective was fulfilled by combining two carbon allotropes, Graphene sheets and CNTs, to construct an entirely novel material, Pillared Graphene. In this architecture, we can tune the pores at will, thus making the material ideal for several applications including hydrogen storage.
By doping Pillared Graphene with alkali cations, we predicted that the hydrogen storage capacity of the material is enhanced. At room temperature and low pressures, required for most applications, the material satisfies the DOE’s technical targets for hydrogen storage.
Hydrogen Storage in Metal Organic Frameworks
Metal Organic Frameworks (MOFs) ) is a novel family of nanoscale framework materials with tunable porosity. MOFs have been synthesized only in the past few years. Since their discovery, these materials have been considered good candidates for hydrogen storage.
Our research aims to enhance the hydrogen storage capacity of MOFs through doping and functionalization. Multi–scale theoretical studies have been performed to identify the factors that affect hydrogen adsorption on MOFs. This knowledge can serve as the key to further strategic optimization of these materials.
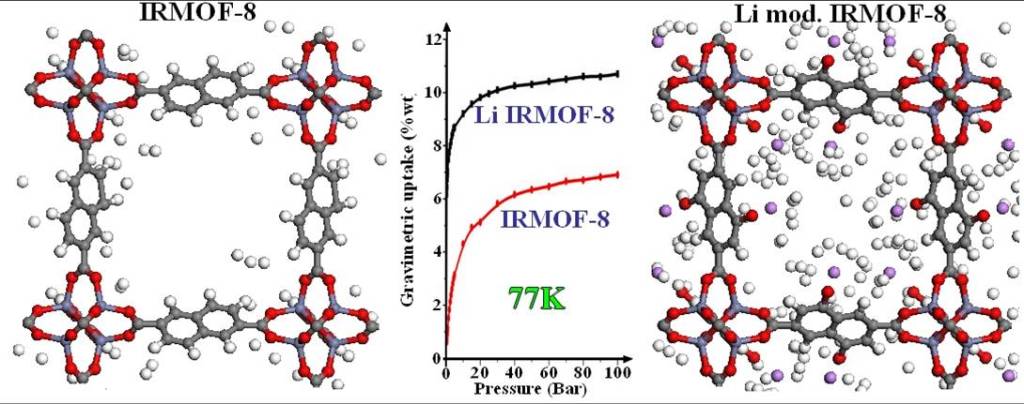
Our 1st strategy is the insertion of point charges in the molecular framework. This can be done by doping the framework with alkali cations, resulting in increased hydrogen storage performance, especially at room temperature and low pressures, as required for most target applications.
Our 2nd strategy is the functionalization of the framework’s organic part. This procedure is experimentally more feasible and gives the same impressive results like the 1st. In the figure above it is visible even with the eye the increasing of the absorbed hydrogen in the functionalized MOF.
In both procedures the predicted amount of stored hydrogen exceeded the gravimetric and volumetric targets that have been set for commercialization.
CO2 Capture & Storage
Carbon dioxide (CO2) is a greenhouse gas that has been directly linked to the global warming problem. Nowadays, many governments and organizations are taking measures and implement new policies in order to limit the extent of CO2 release to the atmosphere.
It is well known that some porous materials can be used, in principle, to capture and store CO2. Nevertheless, the efficient and economically viable capture and storage of CO2 remains an unsolved problem.
Our research is focusing on studying existing nano–porous materials for CO2 capture and storage, as well as on designing novel materials with enhanced performance.
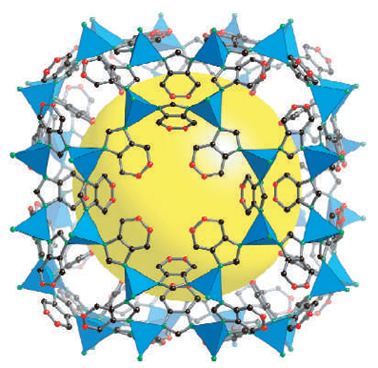
The CO2 storage capacity of many families of porous materials has been tested via computer simulations. We showed that a new class of materials, the Zeolithic Imidazolate Frameworks (ZIFs), is capable of adsorbing large amounts of gas.
High–accuracy quantum chemistry methods were used to understand the nature of the interactions of CO2 with porous adsorbents. The reasons for the enhanced CO2 storage capacity of ZIFs were identified. Based on this progress, further research is underway, focusing on the design of novel nanomaterials for CO2 storage.

