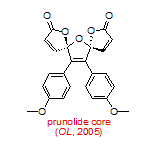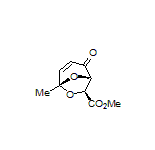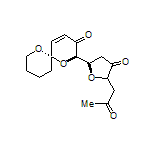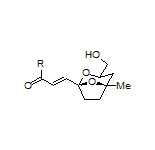Research
..It's not what you make, but how you make it that matters most.
How does one go about improving the traditional labourious stepwise approach to constructing molecules? There are many answers to this question, each with its own validity; our own answer can be seen by looking at the work we do (see, our Publications List).
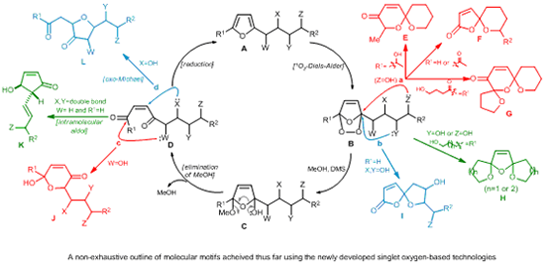
To summarise, we have opted to focus our work mostly in the core area of synthesis of polyoxygenated bioactive molecules. The challenges defined by the concept of ideal synthesis (see, our homepage introduction) are all the more marked here, in a field where chemists frequently find themselves embroiled in protracted protecting group strategies, extensive redox-shuttling (going up and down oxidation states during molecular manipulations) and the use of un-green oxidants (heavy metals and/or heavy molecular weight oxidants). To achieve the desired improvements, we seek to use cascade reaction sequences (to give rapid increases in molecular complexity) mediated by the highly selective (protecting groups are therefore rendered, essentially, unnecessary) and green (it is generated from air by exposure to visible spectrum light in the presence of catalytic natural sensitizers and can be used in water) oxidant, singlet oxygen (1O2).1,2
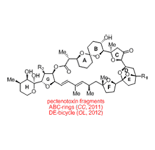
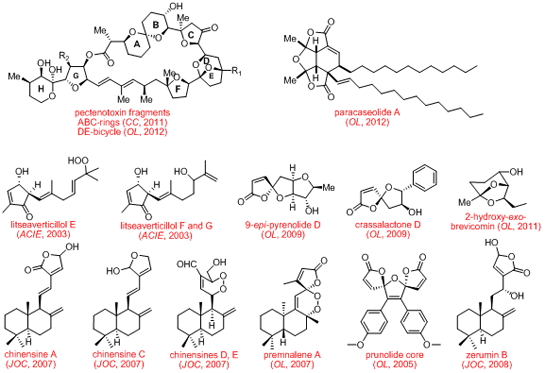
Using all these principles we have, for example, made the important molecular motifs shown below from extremely simple and readily accessible starting substrates.
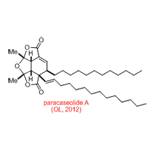
In terms of natural product synthesis, we have made the litseaverticillols (A-G, I, J), chinensines A-E, (+)-premnalane A, crassalactone D, 2-hydroxy-exo-brevicomin, and paracaseolide A, as well as, key complex motifs from the prunolides, pectenotoxin, salinomycin, and spirolide/pinnatoxin/pteriatoxin and analogues from the pyrenolide family (see, our Publications List for details of how we achieved these tasks).
[1] D. Noutsias, I. Alexopoulou, T. Montagnon, G. Vassilikogiannakis Green Chem., 2012, 14, 601.
[2] T. Montagnon, M. Tofi, G. Vassilikogiannakis Acc. Chem. Res. 2008, 41, 1001.



.png)
.png)
.png)
.png)
.png)